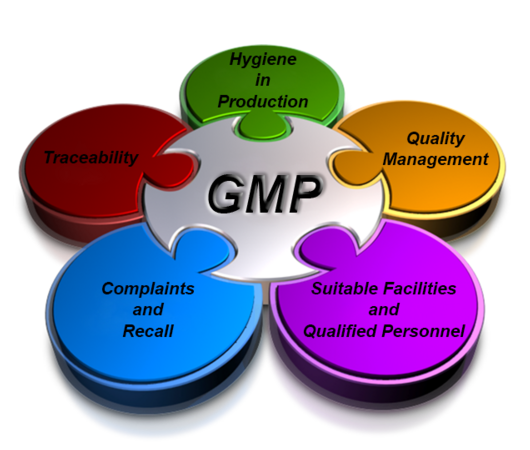

GMP
(Compliance Certification to Pharma & Food Products) Good Manufacturing Practice (GMP) is a term that is recognized worldwide for the control and management of manufacturing, testing and overall quality control of food and pharmaceutical products.
GMP is a good business tool, which will help to refine both compliance and performance of the Company. GMP requirements are largely common sense practices, which will help companies better itself as it moves toward a quality approach using continuous improvement.
